InDex Pharmaceutical’s key focus is to start phase III development of the drug candidate cobitolimod as soon as possible, on the back of the successful results in the phase IIb study CONDUCT, positive regulatory responses from FDA and EMA that endorsed advancement of cobitolimod into phase III studies in patients with moderate to severe left-sided ulcerative colitis, the supportive findings in market research commissioned by InDex, and support from leading European and North American gastroenterologists regarding the potential of cobitolimod.
Phase III is the final stage of drug development before application for market approval by regulatory authorities.
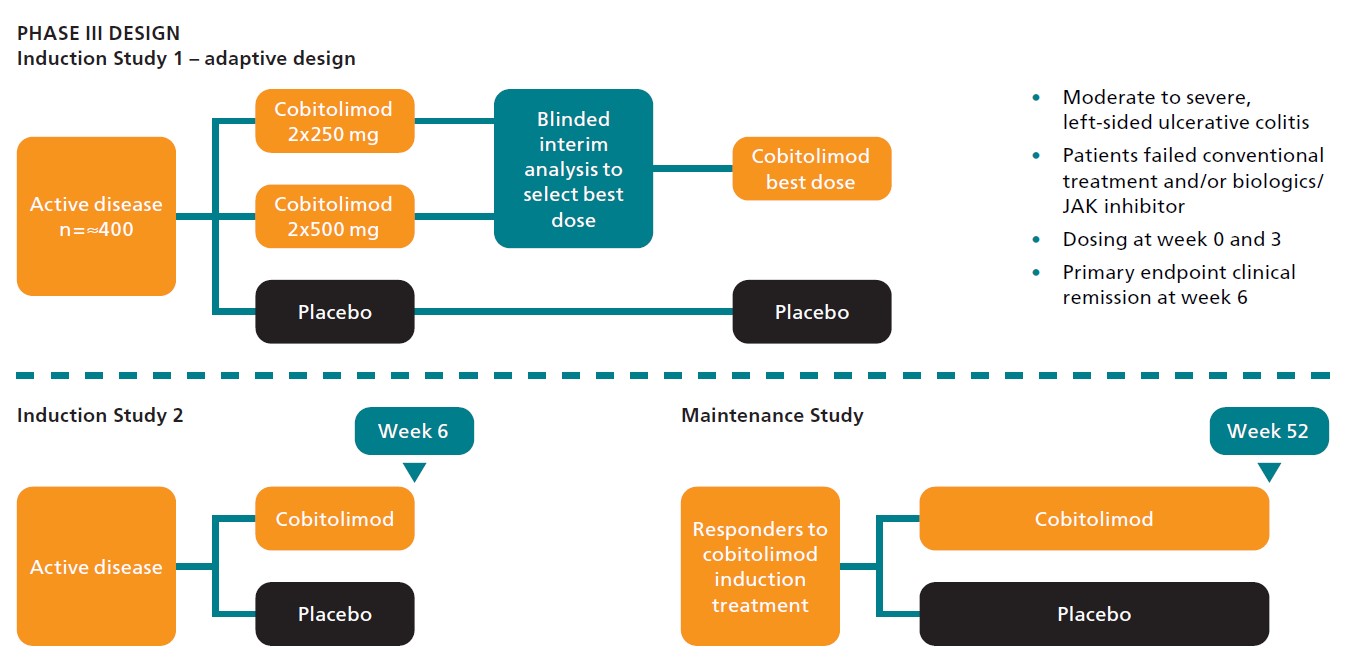
Based on guidance from the FDA and EMA, InDex is planning a sequential phase III program with two induction studies and a yearlong maintenance study with patients that have responded to cobitolimod as induction therapy. The phase III program will form the basis for market approval by confirming cobitolimod’s overall efficacy and safety profile in a large enough group of patients with moderate to severe, left-sided ulcerative colitis with an inadequate response or failure to tolerate conventional therapy, biological therapy or JAK inhibitors.
The important initial phase III induction study, where the primary endpoint of clinical remission will be measured at week 6, will include approximately 400 patients. Apart from the dosing 250 mg x 2, which was the highest dose and the one that showed the best efficacy in the phase IIb study CONDUCT, cobitolimod’s excellent safety profile allows to also evaluate a higher dose, 500 mg x 2. This higher dose has the potential to provide an even better efficacy than what was observed in the CONDUCT study.
When a sufficient number of the participants in the study have been randomized and have eligible data for the primary endpoint, an interim analysis will be performed in a blinded fashion to select the best dose of cobitolimod and the other dose will be dropped. Following the blinded interim analysis, the additional patients to be randomized into the study will receive only the best dose of cobitolimod or placebo. This is referred to as an adaptive study design.
Trial participants will receive a double-blind treatment with cobitolimod or placebo. This means that neither the participant, their treating physician, trial staff, contract research staff nor InDex knows which treatment is being administered. All study medication will be identical regarding appearance, packaging and labeling. The trial will be held blinded until all data has been confirmed and a so-call “clean file” has been created. Only then will the results be compiled according to treatment group.
It will be a global study that includes at least a few hundred clinics. If you are interested in more information about the study, please contact Clinical Trial Manager Karin Arnesson.
Upon a positive read-out of the first study, InDex plans to initiate the second induction study with the best dose. With this sequential design we will read out the outcome of the first induction study before the next study is started, which will reduce the development risk of the program. The results of the first induction study will constitute a significant value inflection point and the remaining program can be optimized accordingly.